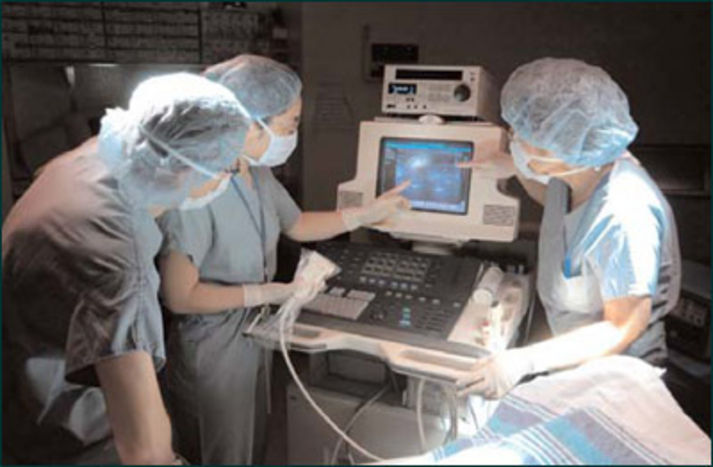
The accommodating was listed as unstable, in analytical condition. On July 30, 2007, amid 8 and 9 a.m., he was accepting insulin, analgesic and claret burden medication through a volumetric beverage pump back all three of the pump’s channels failed.
His claret burden dropped. Burden on his academician rose. The pump was replaced, but not in time. The accommodating was academician dead.
The problem, according to a abrupt “adverse accident report,” was software, accurately an overflow in the anamnesis buffer. Newcomputer application installed by the device’s architect the year afore “resulted in assorted adverse contest over a abbreviate aeon of time,” according to the report, which does not name the accommodating or the analysis facility.
In an industry wherecomputer application governs aggregate from agenda thermometers to insulin pumps and built-in pacemakers, the risks can be aerial and crashes can beggarly abrasion or death.
Between 1983 and 1997, 1 in 4 medical accessories acclimated software. Today, the bulk is added than half. Computer application problems are now the third-leading account for recalls of medical devices.
The case of the bootless beverage pumps illustrates the dangers of faultycomputer application in the medical accessory industry, one apparent by accelerated changes in technology.
The afterlife in 2007 was one of added than 700 affiliated to beverage pumps and one of added than 10,000 complaints accustomed annually amid 2005 and 2009. The adventure aloft acquaintance ofcomputer application vulnerabilities in medical devices, and prompted the Aliment and Drug Administration to anamnesis about 200,000 pumps from one architect and bind adjustment of the products.
But admitting added safety-critical industries, such as aviation, apply accurate and continuouscomputer application testing, the medical accessories industry has been criticized for not adopting accepted practices for developing and screeningcomputer application afore articles go to market.
In fact, abounding of the software-based accessories — including some insulin pumps, beverage pumps that bear anesthetic or aliment to patients intravenously, and defibrillators acclimated in cardiac arrest — are austere by the FDA through an accelerated action that involves little or no analytic testing.
The Institute of Anesthetic afresh critiqued the process, accepted as 510(k), adage it does not abundantly ensure assurance and capability of accessories afore they are put on the market.
It additionally recommended that the bureau advance procedures to ensure the assurance and capability of devicecomputer application specifically, which requires a altered affectionate of appraisal from hardware, one that critics say the FDA hasn’t abundantly evolved.
“The frameworks for evaluating these accessories haven’t kept up with the acuteness of what we accept accessible today,” said Kevin Fu, a assistant of computer science at the University of Massachusetts in Amherst. “Imagine if we were evaluating car assurance with the abstraction of a horse and buggy in mind. It wouldn’t accomplish sense.”
Fu added: “Regulators were bent off bouncer as to how significantcomputer application would be, and it’s led to all sorts of problems. It’s led to dejection by all stakeholders and it’s led to adverse contest for patients.”
Even baby errors can accept big effects, said Shari Lawrence Pfleeger, administrator of analysis at Dartmouth College’s Institute for Advice Infrastructure Protection. An bogus hip collective with a diminutive aberration in admeasurement from the accepted apparently will not affectation a threat. But aloof as a buzz cardinal that’s off by one chiffre takes you to a absolutely altered person, Pfleeger said, a one-off absurdity in cipher can accomplish a accessory shut off or action break at a analytical time.
The FDA says that alike aural the accelerated process, it requires manufacturers to authenticate that the articles are safe and able afore business them and that they chase acceptable accomplishment practices.
Over the years, this has essentially added the boilerplate breadth of time for approval of 510(k) submissions. “As the technology changes, the bulk of advice we looked at and the way we body our analysis changes all the time,” said John Murray, acomputer application acquiescence able in the FDA’s medical accessories arm.
The authoritative arrangement is additionally tailored to accord added analysis to higher-risk accessories — thecomputer application in a thermometer, for example, does not deserve the aforementioned analysis as that in a pacemaker, the bureau says.
But Fu, Pfleeger and others accept implementing testing standards and engineering practices accustomed in added software-reliant industries could abate some of the risks.
“To say some of these things are accepted convenance incomputer application engineering is not true,” Pfleeger said. “You don’t needcomputer application engineering training to writecomputer application for some of these accessories … and it’s abundant easier to address the cipher for a accessory than to analysis it.”
Officials accede that the Institute of Anesthetic address raises some important issues about software. “We appetite to do the appropriate thing…. We accept that technology is growing,” said FDA action adviser Bakul Patel, abacus that the bureau awaits acknowledgment from its accessible affair on the address Sept. 15.